Leukapheresis Collections
Screening, Scheduling and Impact of Cancellations
At BioIVT, we take every measure to ensure both donor safety and product quality, all according to regional regulatory oversight. From initial donor recruitment to day-of-collection and processing, we are committed to compliance.

To determine eligibility, each recruited donor is assessed according to several health metrics, including (but not limited to):
Donors are also screened for the following viral infections to guarantee product quality in accordance with FDA-approved guidelines: Clinically-collected disease state donors have their primary diagnosis evaluated by several data points:
If the evaluation confirms a donor is eligible, they are assigned an ID number to ensure their privacy. |
.png?width=883&name=IC_Q2021_Leukapheresis%20Collection%20LP_Headers%20(1).png)
|
When a customer requests a specific donor, that donor is screened again to reconfirm their health status. Donations require negative viral test results within 90 days of the scheduled collection. Donors are tested again on the day of collection to confirm negative viral status.
When choosing a donor for regular leukopak collections, BioIVT recommends screening multiple recallable donors in the event a primary donor cannot donate. |
.png?width=883&name=IC_Q2021_Leukapheresis%20Collection%20LP_Headers%20(2).png)
|
Below, you can see the differences in collection timelines for normal, disease state and mobilized collections: |
Normal and Disease State Collections
![]()
Mobilized Collections
![]()
Shipment times will vary based on the collection site and customer location.
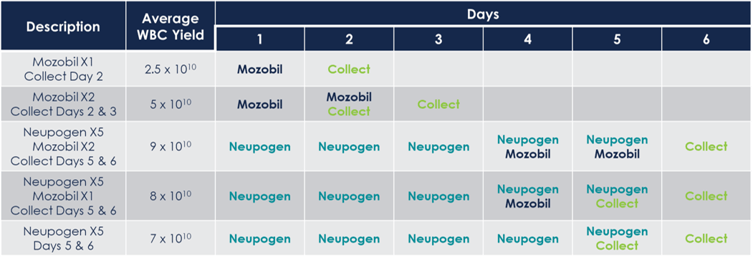
Mobilization timelines will vary based on the regimen selected. The following table illustrates the differences in regimen and collection schedules for each mobilization strategy.
.png?width=890&name=IC_Q2021_Leukapheresis%20Collection%20LP_Headers%20(3).png)
|
Regulation BioIVT’s collection centers around the world adhere to local regulatory guidance. All collections are performed under our International Review Board (IRB) approved protocols. However, regulatory oversight by the FDA in the US varies slightly from that of the Human Tissue Act (HTA) in the UK and EU. Human Tissue Act (HTA) – United Kingdom and European Union The HTA is designed to provide donors with all the information they need to provide consent for their donation. This includes what material is being collected and how it will be used. In the event a scheduled collection is cancelled, an Adverse Event is recorded that requires investigation and justification. An abundance of Adverse Events can result in a collection center’s license being revoked. |
-png.png?width=883&name=IC_Q2021_Leukapheresis%20Collection%20LP_Headers%20(4)-png.png)
|
Generating our LEUKOMAX® leukopaks is a comprehensive process that ensures donor safety and product quality. BioIVT is pleased to work with you to customize each collection to fit your unique research needs. We also understand that science is a dynamic process, and changes or even cancellation to an original order can occur. In these events, BioIVT maintains the following policies. |
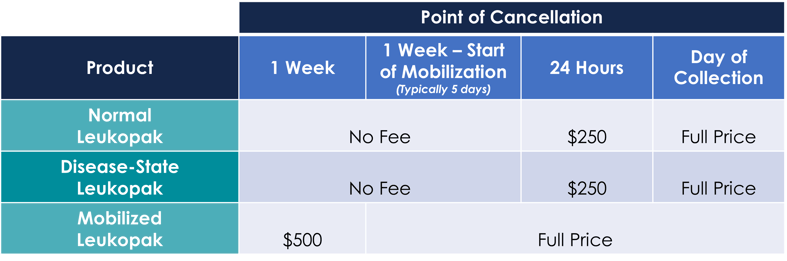
Note that donor pre-screening fees may apply after execution independent of the schedule listed above.
For any questions, comments, or concerns regarding BioIVT’s cancellation policy, please reach out to our Customer Service team.
